Is Amox-clav 875-125 Mg a Family of Penicillin
 | |
 | |
| Combination of | |
|---|---|
| Amoxicillin | Penicillin antibiotic |
| Clavulanic acid | Beta-lactamase inhibitor |
| Clinical data | |
| Merchandise names | Augmentin, Clavulin, Amoclan, others[i] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685024 |
| License information |
|
| Pregnancy category |
|
| Routes of administration | By rima oris, intravenous[2] |
| ATC code |
|
| Legal status | |
| Legal condition |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
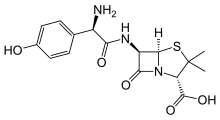
| Formula | C 24 H 27 K N 4 O x S |
| Molar mass | 602.66 g·mol−1 |
| 3D model (JSmol) |
|
| SMILES
| |
| InChI
| |
| | |
Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, is an antibiotic medication used for the treatment of a number of bacterial infections.[3] Information technology is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor.[iii] It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites.[three] Information technology is taken by mouth or by injection into a vein.[2]
Mutual side effects include diarrhea, vomiting, and allergic reactions.[3] It also increases the risk of yeast infections, headaches, and blood clotting problems.[two] [four] Information technology is not recommended in people with a history of a penicillin allergy.[two] It is relatively prophylactic for utilise during pregnancy.[3]
Amoxicillin/clavulanic acid was approved for medical use in the United States in 1984.[3] It is on the Earth Wellness Organisation'southward List of Essential Medicines.[5] The Globe Health System classifies amoxicillin/clavulanic-acid every bit critically important for human medicine.[6] It is available as a generic medication.[3] In 2019, it was the 93rd nigh commonly prescribed medication in the United States, with more than 81000000 prescriptions.[7] [8]
Medical uses [edit]
Amoxicillin/clavulanic acid is widely used to treat or prevent many infections acquired by susceptible leaner, such as:
- urinary tract infections
- respiratory tract infections
- skin and soft tissue infections
- sinus infections
- tonsillitis
- cat scratches
- infections caused by the bacterial flora of the mouth, such as:
- dental infections
- infected animal bites
- infected human bites (including unproblematic "clenched-fist" or "reverse-bite" injuries)[ix] [10]
Information technology is also used for tuberculosis that is resistant to other treatments.[3]World wellness organisation recommends giving amoxicillin-clavulanate along with meropenem as one of the therapeutic options in drug resistant tuberculosis, where clavulanate and non amoxicillin is beingness relied upon for anti TB activity. Withal, across the spectrum of dosage of amoxicillin-clavulanate combination, the dose of clavulanate is abiding at 125 mg, whereas the dose of amoxicillin varies at 250 mg, 500 mg and 875 mg. Thus the apply of depression dose amoxicillin-clavulanate in combination with meropenem may be used in part of treatment authorities for drug resistant TB and this has been demonstrated in a clinical setting also.[11]
This combination results in an antibiotic with an increased spectrum of action and restored efficacy against amoxicillin-resistant bacteria that produce β-lactamase.[ citation needed ]
Adverse effects [edit]
Possible side effects include diarrhea, vomiting, nausea, thrush, and pare rash. These do non usually require medical attending. As with all antimicrobial agents, antibiotic-associated diarrhea due to Clostridium difficile infection—sometimes leading to pseudomembranous colitis—may occur during or afterwards treatment with amoxicillin/clavulanic acid.[10]
Rarely, cholestatic jaundice (too referred to every bit cholestatic hepatitis, a class of liver toxicity) has been associated with amoxicillin/clavulanic acid. The reaction may occur up to several weeks subsequently treatment has stopped, and unremarkably takes weeks to resolve. It is more than frequent in men, older people, and those who have taken long courses of treatment; the estimated overall incidence is one in 100,000 exposures.[ten] In the United Kingdom, co-amoxiclav carries a alarm from the Committee on Prophylactic of Medicines to this effect.[ix]
As all aminopenicillins, amoxicillin has been associated with Stevens–Johnson syndrome/toxic epidermal necrolysis, although these reactions are very rare.[10] [12]
History [edit]
British scientists working at Beecham (at present office of GlaxoSmithKline), filed for US patent protection for the drug combination in 1979. They marketed information technology nether the trade name Augmentin.[9] A patent was granted in 1985.[13] [14]
Preparations [edit]
Amoxicillin/clavulanic acid is the International Nonproprietary Proper name (INN) and co-amoxiclav is the British Canonical Proper noun (BAN).[ commendation needed ]
Many branded products bespeak their strengths as the quantity of amoxicillin. Augmentin 250, for example, contains 250 mg of amoxicillin and 125 mg of clavulanic acid.[ix] [fifteen]
An intravenous preparation has been available in the UK since 1985,[16] but no parenteral training is available in the US;[ citation needed ] the nearest equivalent is ampicillin/sulbactam.[ citation needed ]
Suspensions of amoxicillin/clavulanic acid are available for utilise in children.[ citation needed ] They must be refrigerated to maintain effectiveness.[ citation needed ]
Veterinary use [edit]
Amoxicillin/clavulanic acid is used in numerous animals for a variety of conditions:
- Dogs: periodontitis, kennel cough[17] [xviii]
- Cats: urinary tract infections, skin and soft tissue infections[ citation needed ]
- Calves: enteritis, navel ill[ citation needed ]
- Cattle: respiratory tract infections, soft tissue infections, metritis, mastitis[ citation needed ]
- Pigs: respiratory tract infections, colibacillosis, mastitis, metritis, agalactia[ commendation needed ]
In combination with prednisolone, information technology is used for intramammary infusion for the treatment of mastitis in lactating cows.[ citation needed ] Merchandise names include Clavaseptin, Clavamox, and Synulox.[ citation needed ]
Amoxicillin/clavulanic acrid is banned from apply in domestic-food animals (cattle, swine, etc.) in both the Us and Europe;[ citation needed ] in the Great britain, Synulox tin can be used in domestic-nutrient animals as long as a specified withdrawal menses is observed.[ commendation needed ]
Bacterial resistance [edit]
Bacterial antibiotic resistance is a growing problem in veterinary medicine. Amoxicillin/clavulanic acid is reported to be effective against clinical Klebsiella infections, just is non efficacious against Pseudomonas infections.[19]
References [edit]
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 97. ISBN9781284057560.
- ^ a b c d Globe Health Arrangement (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 102. hdl:10665/44053. ISBN9789241547659.
- ^ a b c d east f grand h "Amoxicillin and Clavulanate Potassium". The American Club of Wellness-System Pharmacists. Archived from the original on 29 Nov 2016. Retrieved 8 December 2016.
- ^ Gillies, M; Ranakusuma, A; Hoffmann, T; Thorning, S; McGuire, T; Glasziou, P; Del Mar, C (17 Nov 2014). "Common harms from amoxicillin: a systematic review and meta-assay of randomized placebo-controlled trials for whatsoever indication". Canadian Medical Clan Journal. 187 (1): E21-31. doi:10.1503/cmaj.140848. PMC4284189. PMID 25404399.
- ^ World Wellness System (2019). World Health Arrangement model list of essential medicines: 21st listing 2019. Geneva: World Health System. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC Past-NC-SA three.0 IGO.
- ^ World Wellness Organization (2019). Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Wellness Organisation. hdl:10665/312266. ISBN9789241515528.
- ^ "The Top 300 of 2019". ClinCalc . Retrieved sixteen October 2021.
- ^ "Amoxicillin; Clavulanate - Drug Usage Statistics". ClinCalc . Retrieved 16 Oct 2021.
- ^ a b c d British National Formulary (57 ed.). March 2009.
- ^ a b c d Gordon D (2010). "Amoxicillin–Clavulanic Acrid (Co-Amoxiclav)". In Grayson ML; et al. (eds.). Kucers' the Use of Antibiotics: a Clinical Review of Antibacterial, Antifungal, Antiparasitic and Antiviral Drugs. London: Hodder Arnold/ASM Press. pp. 193–iv. ISBN978-0-340-92767-0.
- ^ Mishra, Gyanshankar; Caminero, Jose (2018). "First Successful Use of Depression Dose Amoxicillin-Clavulanic Acid in Management of Drug Resistant Tuberculosis". Journal of Clinical and Diagnostic Research. 12 (10): OD08–OD10. doi:ten.7860/JCDR/2018/37279.12145 . Retrieved vii May 2021.
- ^ Harr T, French LE (2010). "Toxic epidermal necrolysis and Stevens-Johnson syndrome". Orphanet Journal of Rare Diseases. 5: 39. doi:10.1186/1750-1172-5-39. PMC3018455. PMID 21162721.
- ^ U.s. 4441609
- ^ Bryan, Jenny (23 June 2011). "However going strong at 30: co-amoxiclav". The Pharmaceutical Journal. 286: 762.
- ^ "Augmentin -- Prescribing Information" (PDF). December 2006. Archived (PDF) from the original on 20 Dec 2013.
- ^ Davies BE, Boon R, Horton R, Reubi FC, Descoeudres CE (October 1988). "Pharmacokinetics of amoxycillin and clavulanic acrid in haemodialysis patients following intravenous administration of Augmentin". British Journal of Clinical Pharmacology. 26 (four): 385–90. doi:10.1111/j.1365-2125.1988.tb03395.x. PMC1386558. PMID 3190988.
- ^ "Canine Infectious Tracheobronchitis (Kennel Cough)". Archived from the original on 21 May 2006. Retrieved 30 May 2013.
- ^ "Kennel Cough - Symptoms and Treatment". Archived from the original on 10 May 2013. Retrieved 30 May 2013.
- ^ Federation of Veterinarians in Europe Position Paper: "Antibiotic Resistance & Prudent Use of Antibiotics in Veterinary Medicine"
External links [edit]
- "Amoxicillin / clavulanic acid". Drug Information Portal. U.South. National Library of Medicine.
Source: https://en.wikipedia.org/wiki/Amoxicillin/clavulanic_acid
0 Response to "Is Amox-clav 875-125 Mg a Family of Penicillin"
Post a Comment